How long it will take to transition a potential viral vaccine or viral vector product from bench to bed is a common question from clients. What shortcuts may be used to move even more quickly is frequently the following inquiry. Strong preclinical evidence from in vivo studies demonstrating the potential product’s good efficacy is insufficient to permit quick testing on humans. In actuality, it takes a tremendous amount of effort to go from “bench to bed.” For instance, the demanding Good Manufacturing Practices (GMP) criteria are frequently not met by the manufacturing and purification methods utilized during the research phase. Therefore, these procedures require redesign. Furthermore, it is necessary to show that the anticipated production method is reliable. This implies that it should always produce a good product with a specific quality range.
You need to be an expert in regulatory standards if you want to build a production method that complies with GMP virus production. At Batavia, the group that creates and refines a production method in an R&D setting also produces the finished good under GMP. This configuration makes sure that the engaged team considers GMP rules while creating a procedure. Additionally, it has been demonstrated that this strategy avoids risky and time-consuming tech transfer processes from development into GMP. As a result, there are no or few variances in production batch records, and there is an exceptional track record of successful GMP manufacturing campaigns.
What is GMP?

A method known as good manufacturing practice (GMP) is used to guarantee that goods are consistently manufactured and monitored in accordance with quality standards. It is intended to reduce any production-related risks associated with pharmaceuticals that cannot be avoided through testing the finished product. The main risks include unanticipated product contamination, which can harm health or even result in death; inaccurate labeling on containers, which could result in patients receiving the wrong medication; and an insufficient or excessive amount of active ingredient, which can lead to ineffective treatment or negative effects. GMP includes all area of production, including raw materials, space, and tools, as well as employee training and personal hygiene. Every process that can have an impact on the final product’s quality requires specific, defined procedures. Systems must exist to show written evidence that the right steps are consistently taken at each stage of the manufacturing process, each time a product is manufactured. WHO has laid out specific recommendations for optimal manufacturing practice. Based on WHO GMP, many nations have created their own GMP regulations. Others, such as the Association of South-East Asian Nations (ASEAN), the European Union, and the Pharmaceutical Inspection Convention, have standardized their criteria.
Major challenge when producing biotics under GMP

Evaluating AMs’ properties can be difficult because many of them are intricate biological materials. The complexity of biologics and the use of cutting-edge technologies provide difficulties for cGMP compliance. The FDA’s GMP regulations are generally not difficult to follow, but there are a few nuances that could be a challenge. For instance, it is straightforward to perform a gap analysis and validate that procedures are compliant with the numerous rules, specifications, and technical documents that make up modern CGMPs. However, the amount of intricate formulation work needed for biological products is rather difficult.
Since biologics are made from living things, which are inherently unpredictable, they are challenging to work with. As a result, maintaining consistent product quality, which depends on both entering raw materials and manufacturing processes, is the most challenging task from a GMP perspective. Consistency becomes more difficult when more complexity is present, such a non-standard dosing form.
Challenges in the production of viruses
I’ll go over four instances to illustrate the difficulties a CDMO faces when producing virus-based goods in a GMP setting.
1. Beginning supplies
The first illustration concerns the supplies that were employed, such as the starting material, disposables, medium, and buffers. Fully traceable materials are used in processes that adhere to GMP standards. This means that any plasmids, viral seeds, cell lines, or other raw materials utilized in the procedure must comply with GMP and be accompanied by certificates attesting to their high quality for things like sterility, mycoplasma, and TSE/BSE.
2. Sanitized verification
The second illustration is that not all viral products can be processed downstream using sterile filtering. The measles virus is one example of a virus that is relatively big and prevents the adoption of a final sterile filter. Therefore, the creation of measles virus seeds requires an aseptic procedure. Before beginning the actual manufacture of virus seeds, an aseptic process needs to be validated. The full USP and DSP processes are both simulated during the validation procedure. The simulation procedure uses bacterial growth-promoting medium, such as TSB, in place of regular medium. Typically, a method is considered validated after producing at least three batches in a row. This entails testing the full processed volume as well as a growth promotion test to validate the medium’s capacity for growth promotion.
3. D.s (Individual Defense Equipment)
There is frequently an added challenge when working with biological agents that have a higher biosafety level in a GMP environment. In these circumstances, you must take strict safety procedures to protect the workers and the environment from contamination in addition to working under GMP guidelines to safeguard the product. For instance, the GMP requirements are not always met by the mandatory Personal Protective Equipment (PPE) needed for dealing with the poliovirus. PPE generally protects the user from exposure, and GMP-compliant gowning materials reduce the introduction and creation of particles inside the cleanroom. Mouth masks and gowns are two examples of PPE that provide this protection. As a result, creating a gowning procedure that satisfies both GMP and biosafety criteria is difficult.
4. Biosafety
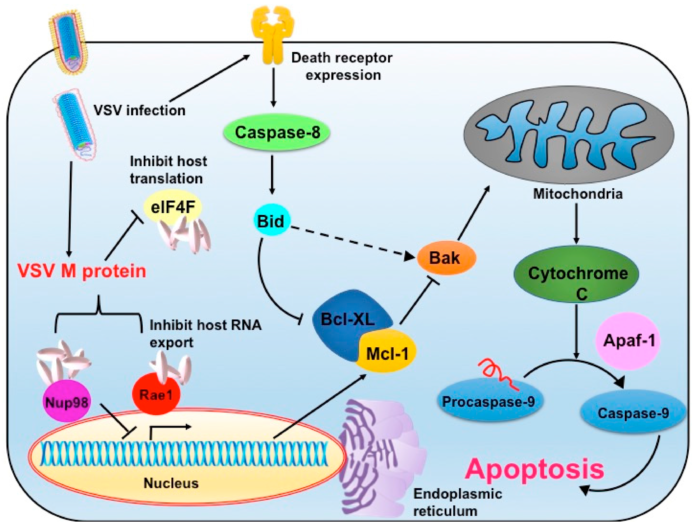
The vesicular stomatitis virus (VSV) vector is the final illustration. Because of its oncolytic qualities, this vector is becoming more and more popular. The local virus, however, poses a serious threat to cattle. Therefore, a vesicular stomatitis virus outbreak could result in large financial losses. The cleanroom must therefore be fumigated after handling the vesicular stomatitis virus as one of the requirements. Cleaning the entire facility is required before beginning any additional production campaign since the environmental monitoring of the cleanroom and the gowning areas are significantly impacted by the H2O2 fumigation of the cleanroom.
It’s essential to have a solid understanding of regulatory standards if you want to manufacture therapeutic products based on viruses with a high success rate. We consistently go above and above to reduce hazards for our clients. Due to the smooth transfer to the clinic, everything goes according to plan and budget.









